DALLAS, TX -- October 2nd, 2024 -- Incannex Healthcare Inc. (NasdaqGM:IXHL): Stonegate Capital Partners initiates their coverage on Incannex Healthcare Inc. (NasdaqGM:IXHL)
Business Overview
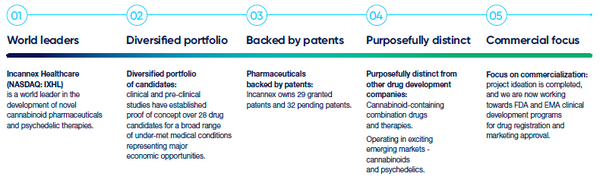
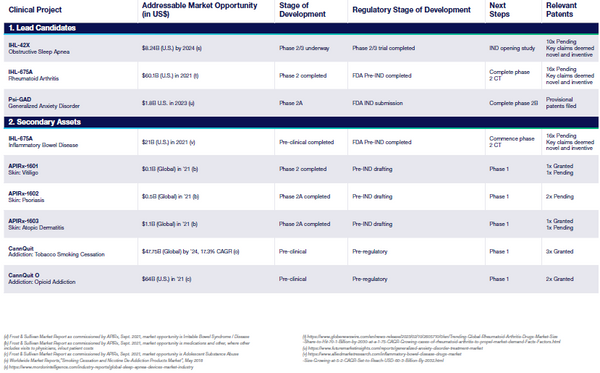
Incannex Healthcare, Inc. (“the Company”, “Incannex”, or “IXHL”) is a clinical-stage biopharmaceutical development company focused on developing innovative medicines for patients living with chronic diseases and significant unmet need. Incannex is advancing proprietary, synthetic first- and best-inclass cannabinoid and psychedelic-assisted therapeutics targeting sleep apnea, anxiety, and inflammatory diseases. Incannex's lead programs include IHL-42X for the treatment of obstructive sleep apnea (OSA), PSX-001 in development to assess the use of psilocybin combined with psychological therapy for generalized anxiety disorder (GAD), and IHL-675A for rheumatoid arthritis (RA). Each of these programs target conditions for which there are either no approved treatments or the available treatments are inadequate. In 2023 IXHL re-domiciled from Australia to Delaware, with a continued listing on NASDAQ under the ticker symbol “IXHL”.
Highlights
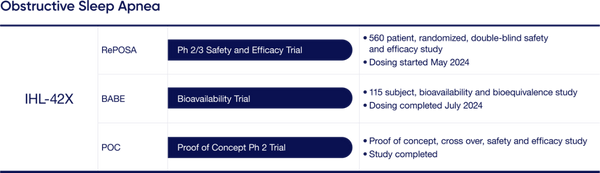
- IHL-42X Update: IHL-42X is a novel treatment designed to treat people suffering from Obstructive Sleep Apnea (OSA) which is characterized by interrupted breathing while asleep. Most recently the Company has initiated dosing for the Phase 2/3 FDA trial. This trial will encompass 560 patients in a randomized double-blind safety and efficacy study. Top line results are expected in the first half of 2025
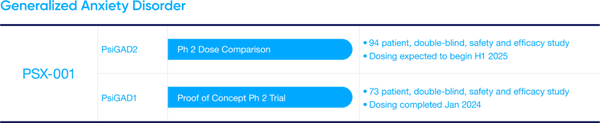
- PSX-001 Update: PSX-001 is Incannex’s psilocybin drug product designed for use with psychological therapy to treat people suffering from Generalized Anxiety Disorder (GAD). Most recently the Company has announced positive top line results from the Phase 2 proof of concept trial completed in Australia. The psilocybin and psychotherapy combination was observed to significantly reduce anxiety scores in patients. Additionally, IXHL has received IND clearance from the FDA, further derisking the asset.
- IHL-675A Update: IHL-675A is a novel treatment designed to treat people suffering from inflammation which is a major contributing factor to rheumatoid arthritis. Most recently the Company completed dosing in an Australian phase 2 clinical trial. This trial is planned to include approximately 128 subjects, with top line date expected in the second half of 2025.
Valuation
- We use a probability-adjusted Discounted Cash Flow Model when valuing IXHL. Our valuation model returns a valuation range of $4.93 to $5.83 with a midpoint of $5.37 based on a discount rate range of 12.25% to 12.75% and a current risk adjustment range of 14% to 16%. Further details on our model can be found on page 5 of this report. We note that this model is highly levered to the out years due to the long term nature of IXHL's industry, leading to the potential for dramatic re-ratings as new information becomes available.