DALLAS, TX -- June 2nd, 2023 -- Bio-Path Holdings, Inc. (Nasdaq:BPTH): The full report can be accessed by clicking on the following link: BPTH Q1 23 Report
Awaiting Topline Results in Several Key Cohorts
- Ongoing Clinical Trials: The Company has various product candidates in different stages of development and is currently expecting near-term topline results in key cohorts of its Phase 1/1b study of BP1001-A in solid tumors, its Phase 1/1b study of BP1002 in relapsed/refractory AML, as well as its Phase 2 study of prexigebersen in AML.
- Prexigebersen - Bio-Path has completed Phase 1 clinical trials for its lead candidate prexigebersen for acute myeloid leukemia (AML) and other blood cancers and is in the midst of a Phase 2 clinical trial for AML.
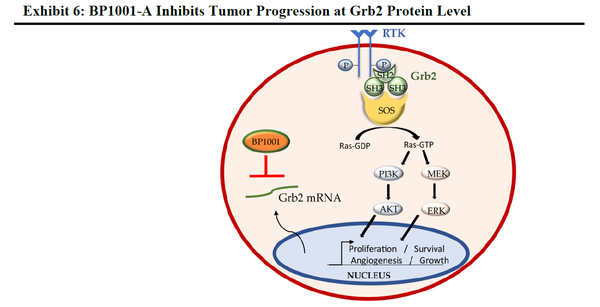
- BP1001-A – BP1001-A (prexigebersen with enhanced nanoparticle properties) has begun Phase 1 trials for the treatment of solid tumors.
- BP1002 – Bio-Path is conducting two clinical trials for BP1002. A Phase 1 clinical trial of BP1002 in patients with advanced lymphoid malignancies is ongoing. Also, a Phase 1 is underway for patients with refractory/relapsed AML, including those who have relapsed from venetoclax-based treatment.
- BP1003 - BP1003 is in pre-clinical development in a pancreatic patient-derived tumor model. In previous preclinical trials, it has been successful at penetrating pancreatic tumors.
- Owning the Breakthrough Technology: Bio-Path has developed a proprietary antisense and liposome delivery technology for DNA drugs, DNAbilize®, potentially solving the challenges of delivering these molecules directly to target cells without side effects. DNAbilize® is Bio-Path’s novel and patented method for producing antisense DNA therapeutics for a broad spectrum of indications, including cancer. This technology overcomes certain drawbacks and challenges of the more traditional methods.
- Strategic Relationships: The original technology platform was licensed from The MD Anderson Cancer Center; BPTH maintains strong relationship with the Cancer Center as well numerous leading cancer centers across the US, with several hosting clinical trials.
- Strong IP Position: Bio-path has a strong IP position with composition of matter and method patents for antisense targets and manufacturing which helps ensure technology preservation and offers protection against competitors.
- Cash Runway: The Company reported $6.7M as cash on hand as of 3/31/22, and management has stated that additional funds will be needed to continue operations according to plan for the upcoming 12 months.
- Valuation: Using comparable companies’ EV/R&D multiple - we gauge that Bio- Path is significantly undervalued next to its peers with numerous drug candidates in the pipeline and its lead drug candidate is quickly approaching milestone indication that could indicate potential for approval. See page 12 for further details.